Very happy to report our recent collaborative research paper published in the Journal of Clinical Investigation (IF 15.9), in a strongly interdisciplinar collaboration with the research group of Prof Patricia Maciel (ICVS, UMinho).
In this paper entitled “Glucocorticoid receptor-dependent therapeutic efficacy of tauroursodeoxycholic acid in preclinical models of Spinocerebellar ataxia type 3” we analyze the therapeutic efficacy of TUDCA in the treatment of Spinocerebellar ataxia type 3 (SCA3), an adult-onset neurodegenerative disease, establishing a novel in vivo mechanism of the neuroprotective effects of this drug in the treatment SCA3 and proposing this drug for clinical trials in patents SCA3 patients.
Glucocorticoid receptor-dependent therapeutic efficacy of tauroursodeoxycholic acid in preclinical models of Spinocerebellar ataxia type 3
Sara Duarte-Silva, Jorge Diogo Da Silva, Daniela Monteiro-Fernandes, Marta Daniela Costa, Andreia Neves-Carvalho, Mafalda Raposo, Carina Soares-Cunha, Joana S. Correia, Gonçalo Nogueira-Goncalves, Henrique S. Fernandes, Stephanie Oliveira, Ana Rita Ferreira-Fernandes, Fernando Rodrigues, Joana Pereira-Sousa, Daniela Vilasboas-Campos, Sara Guerreiro, Jonas Campos1, Liliana Meireles-Costa, Cecilia M. P. Rodrigues, Stephanie Cabantous, Sérgio. F. Sousa, Manuela Lima, Andreia Teixeira-Castro, Patricia Maciel
Journal of Clinical Investigation (2023) | DOI: https://doi.org/10.1172/JCI162246
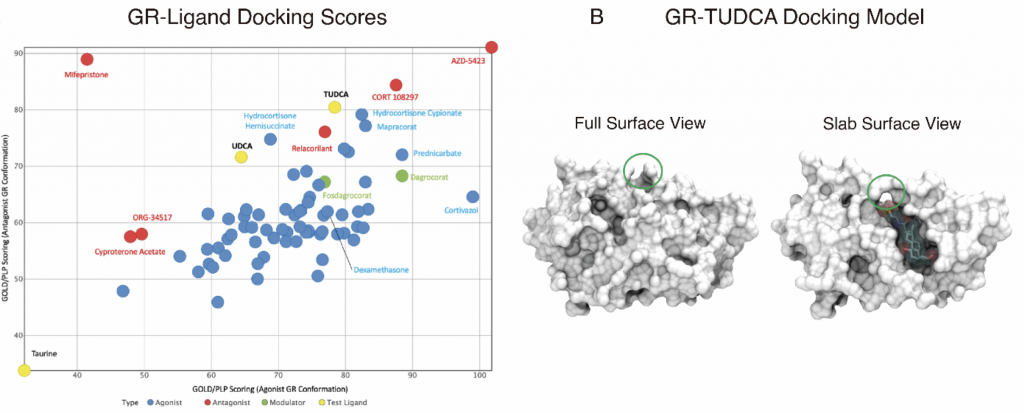
ABSTRACT
Spinocerebellar ataxia type 3 (SCA3) is an adult-onset neurodegenerative disease caused by a polyglutamine expansion in the ataxin-3 (ATXN3) gene. No effective treatment is available for this disorder, other than symptom-directed approaches. Bile acids have shown therapeutic efficacy in neurodegenerative disease models. Here, we pinpointed tauroursodeoxycholic acid (TUDCA) as an efficient therapeutic, improving the motor and neuropathological phenotype of SCA3 nematode and mouse models. Surprisingly, transcriptomic and functional in vivo data showed that TUDCA acts in neuronal tissue through the glucocorticoid receptor (GR), but independently of its canonical receptor, the FXR. TUDCA was predicted to bind to the GR, similarly to corticosteroid molecules. GR levels were decreased in disease-affected brain regions, likely due to increased protein degradation as a consequence of ATXN3 dysfunction, being restored by TUDCA treatment. Analysis of a SCA3 clinical cohort showed intriguing correlations between the peripheral expression of GR and the predicted age at disease onset, in pre-symptomatic subjects, and of FKBP5 expression with disease progression, suggesting this pathway as a potential source of biomarkers for future study. We have established a novel in vivo mechanism for the neuroprotective effects of TUDCA in SCA3, and propose this readily available drug for clinical trials in SCA3 patients.