Happy to report our recent collaborative research paper published in the Journal of Structural Biology (IF 3.234), in a collaboration with the research group of Prof Luis Gales (I3S, ICBAS).
In this paper entitled “Crystal structures of Streptomyces tsukubaensis sigma factor SigG1 and anti-sigma RsfG” we report the X-ray structures of Streptomyces tsukubaensis SigG1and RsfG improving our understanding of their functional mechanism.
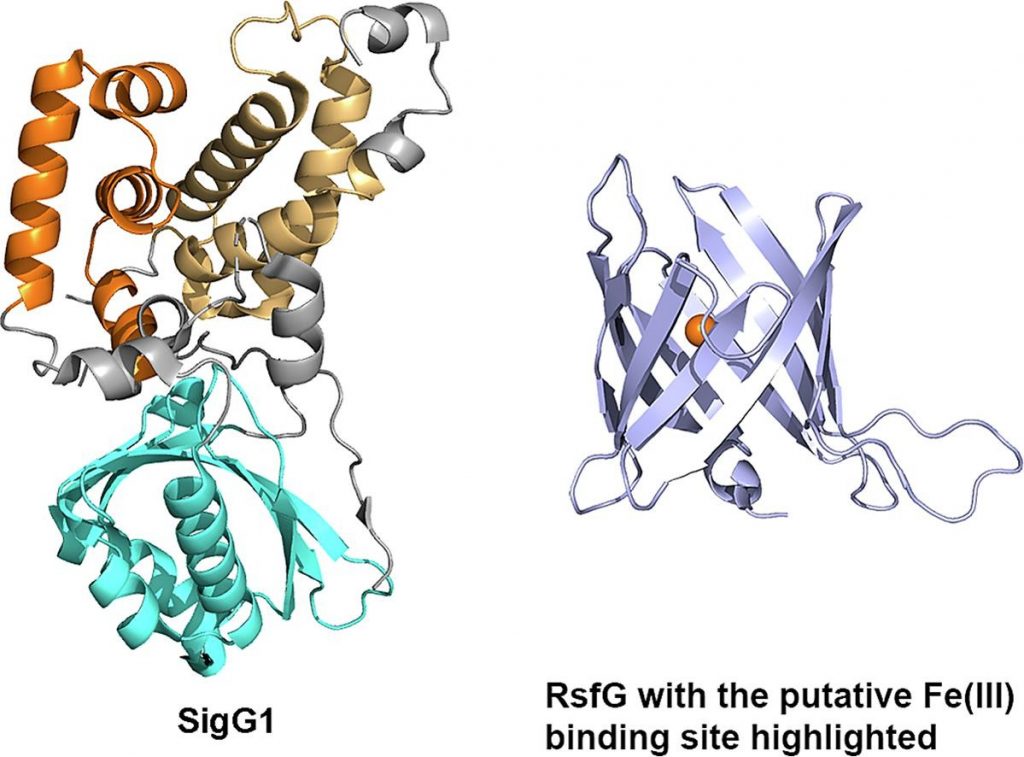
Biomolecular simulations were employed to evaluate possible metal binding motifs inside a rare β-barrel fold observed in the X-ray. Analysis of the metal binding motifs inside the protein barrel by bioinformatics and molecular dynamics simulations are consistent with Fe(III) binding, which is in agreement with previous findings that the Streptomyces tsukubaensis ECF56 SigG1-RsfG system is involved in metal-ion homeostasis.
This putative iron binding site in the interior of the RsfG β-barrel might provide the link between the structure and the protein’s proposed role in metal homeostasis although additional work is needed to disclose the regulatory molecular mechanism of the SigG1-RsfG system.
Crystal structures of Streptomyces tsukubaensis sigma factor SigG1 and anti-sigma RsfG
José P. Leite, Frederico Lourenço, Rute Oliveira, Sérgio. F. Sousa, Marta V. Mendes and Luis Gales
Journal of Structural Biology (2023) | DOI: 10.1016/j.jsb.2023.108038
ABSTRACT
Transcription of specific genes in bacteria under environmental stress is frequently initiated by extracytoplasmic function (ECF) σ factors. ECFs σ factors harbour two conserved domains, σ2 and σ4, for transcription initiation by recognition of the promoter region and recruitment of RNA polymerase (RNAP). The crystal structure of Streptomyces tsukubaensis SigG1, an ECF56-family σ factor, was determined revealing σ2, σ4 and the additional carboxi-terminal domain SnoaL_2 tightly packed in a compact conformation. The structure of anti-sigma RsfG was also determined by X-ray crystallography and shows a rare β-barrel fold. Analysis of the metal binding motifs inside the protein barrel are consistent with Fe(III) binding, which is in agreement with previous findings that the Streptomyces tsukubaensis ECF56 SigG1-RsfG system is involved in metal-ion homeostasis.
Keywords: Crystal structures; ECF56; Streptomyces tsukubaensis; σ/anti-σ pair.