Please check our recent publication on the study of the catalytic mechanism of the Main Protease of the SARS-CoV-2, published in Molecular Diversity (IF 2.013).
New insights into the catalytic mechanism of the SARS-CoV-2 main protease: an ONIOM QM/MM approach
Henrique S. Fernandes, Sérgio F. Sousa, Nuno M.F.S.A Cerqueira
Molecular Diversity | DOI: 10.1007/s11030-021-10259-7
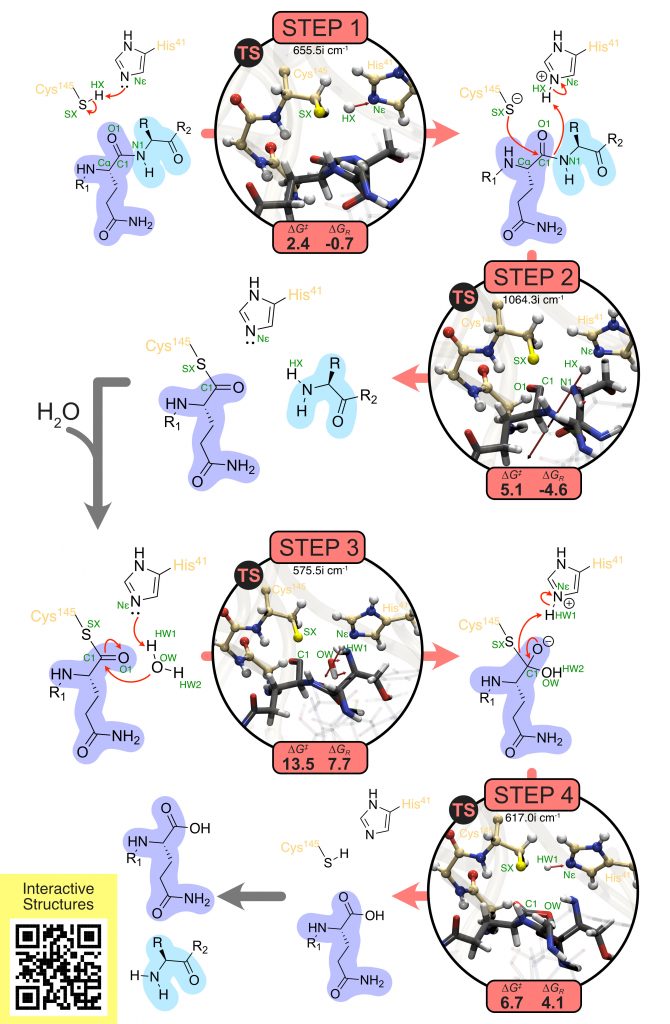
SARS-CoV-2 Mpro, also known as the main protease or 3C-like protease, is a key enzyme involved in the replication process of the virus that is causing the COVID-19 pandemic. It is also the most promising antiviral drug target targeting SARS-CoV-2 virus. In this work, the catalytic mechanism of Mpro was studied using the full model of the enzyme and a computational QM/MM methodology with a 69/72-atoms QM region treated at DLPNO-CCSD(T)/CBS//B3LYP/6-31G(d,p):AMBER level and including the catalytic important oxyanion-hole residues. The transition state of each step was fully characterized and described together with the related reactants and products. The rate-limiting step of the catalytic process is the hydrolysis of the thioester-enzyme adduct, and the calculated barrier closely agrees with the available kinetic data. The calculated Gibbs free energy profile, together with the full atomistic detail of the structures involved in catalysis, can now serve as valuable models for the rational drug design of transition state analogs as new inhibitors targeting the SARS-CoV-2 virus.