Please, check our recent publication on the inhibition of SARS-CoV-2 developed at BioSIM in collaboration with the University of Bordeaux and University of Nantes, already available at bioRxiv:
In silico, in vitro and in cellulo models for monitoring SARS-CoV-2 spike/human ACE2 complex, viral entry and cell fusion
Delphine Lapaillerie, Cathy Chariler, Henrique S. Fernandes, Sergio F. Sousa, Paul Lesbats, Pierre Weigel, Alexandre Favereaux, Veronique Guyonnnet-Duperat and Vincent Parissi
DOI: 10.1101/2021.02.03.429555
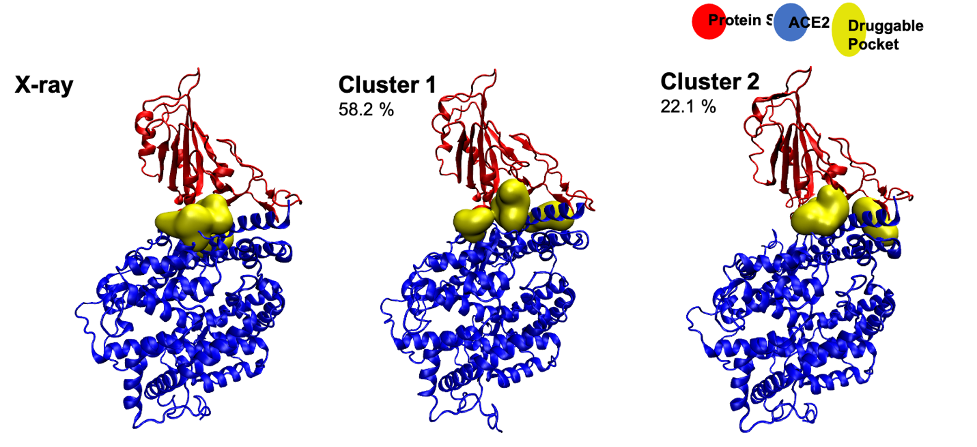
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent responsible for the recent coronavirus disease 2019 (COVID-19) pandemic. Productive SARS-CoV-2 infection relies on viral entry into cells expressing angiotensin-converting enzyme 2 (ACE2). Indeed, viral entry into cells is mostly mediated by the early interaction between the viral spike protein S and its ACE2 receptor. The S/ACE2 complex is, thus, the first contact point between the incoming virus and its cellular target; consequently, it has been considered an attractive therapeutic target.
To further characterize this interaction and the cellular processes engaged in the entry step of the virus, we set up various in silico, in vitroand in cellulo approaches that allowed us to specifically monitor the S/ACE2 association.
We report here a novel computational model of the SARS-CoV-2 S/ACE2 complex as well as its biochemical and biophysical monitoring using pulldown, AlphaLISA and biolayer interferometry (BLI) binding assays. This led us to determine the kinetic parameters of the S/ACE2 association and dissociation steps. In parallel to these in vitro approaches, we developed in cellulo transduction assays using SARS-CoV-2 pseudotyped lentiviral vectors and HEK293T-ACE2 cell lines generated in-house. This allowed us to recapitulate the early replication stage of the infection mediated by the S/ACE2 interaction and to detect cell fusion induced by the interaction. Finally, a cell imaging system was set up to directly monitor the S/ACE2 interaction in a cellular context, and a flow cytometry assay was developed to quantify this association at the cell surface.
Together, these different approaches are available for both basic and clinical research aiming to characterize the entry step of the original SARS-CoV-2 strain and its variants as well as to investigate the possible chemical modulation of this interaction. All these models will help in identifying new antiviral agents and new chemical tools for dissecting the virus entry step.
