Mechanistic Studies of a Flavin Monooxygenase: Sulfur Oxidation of Dibenzothiophenes by DszC
Ana C. C. Barbosa, Rui P. P. Neves, Sérgio F. Sousa, Maria J. Ramos, and Pedro A. Fernandes
DOI: 10.1021/acscatal.8b01877| ACS Catalysis
Abstract
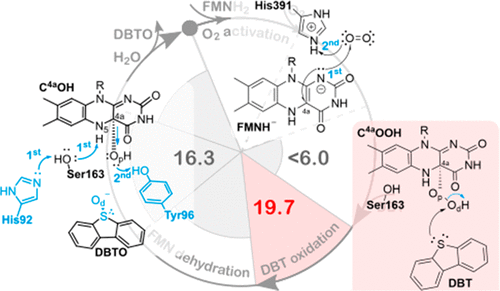
Flavin monoxygenases (FMOs) are enzymes of increasing biotechnological (e.g., crude oil biodesulfurization) and pharmacological (e.g., drug metabolism) interest that perform the oxidation of soft nucleophiles and play key roles in the excretion of xenobiotics or in sulfur amino acid metabolism. DszC is a key FMO involved in sulfur oxidation of dibenzothiophenes (DBTs) through the 4S metabolic pathway of some bacteria. This pathway can be a cheaper and greener alternative for sulfur removal, as DBTs are the major source of crude oil sulfur. Here, we investigate the reaction mechanism of DszC with quantum mechanics/molecular mechanics methods (SCS-MP2/def2-TZVPP:ff10//B3LYP/6-31G(d):ff10). We observe that the reaction mechanism of DBT oxidation occurs in three stages: (1) spin-forbidden formation of a C4a-hydroperoxyflavin (C4aOOH) intermediate; (2) oxidation of DBT to DBTO, upon nucleophilic attack of the DBT-sulfur on the distal oxygen of C4aOOH; and (3) proton transfer from the N5H of the flavin group to the His92-imidazole via Ser163-hydroxyl, releasing a water molecule and oxidized flavin mononucleotide. The overall reaction is computed to be exergonic (−38.7 kcal·mol–1), and the rate-limiting step is the oxidation of DBT to DBTO (ΔG‡ = 19.7 kcal·mol–1, consistent with the experimental turnover of 1.6 min–1). We observe that oxygen activation is a nearly spontaneous process that occurs through a proton-coupled electron transfer to produce a hydroperoxyl radical, followed by a triplet-singlet spin-forbidden inversion to form the C4aOOH intermediate. In agreement with other studies, His391 is a key acid to activate O2 and form the covalent bond. Further clarifying previous mutagenesis results, we also propose that His92 and Tyr96 are key residues for the mechanism: His92 acts as acid to deprotonate N5H in flavin via Ser163; and Tyr96 enhances the oxidation of DBT-sulfur by anchoring the proximal oxygen of C4aOOH, and acts as acid to form the water byproduct and regenerate the flavin cofactor. These are important results to clarify the chemistry of flavin monoxygenases and to open doors for the rational design of DszC mutants with improved catalytic activity.
