Happy to share our new research article published in Journal of Molecular Structure (IF: 3.841), an interdisciplinary collaboration with the research group of Nenad Filipovic and other research groups from University of Belgrad (Serbia) and with researchers from the Universidad de La Laguna (Tenerife, Spain), Marmara University (Turkey), University of Novi Sad (Serbia), Gdansk Technical University (Poland), and Universidad de La Rioja (Spain).
Structural, physicochemical and anticancer study of Zn complexes with pyridyl-based thiazolyl-hydrazones
Jovana B. Araškov, Natalia Maciejewska, Mateusz Olszewski, Aleksandar Višnjevac, Vladimir Blagojević, Henrique S. Fernandes, Sérgio F. Sousa, Adrián Puerta, José M. Padrón, Berta Barta Holló, Miguel Mongi, María Rodríguez-Castillo, José M. López-de-Luzuriaga, Özlem Uğuz, Atıf Koca, Tamara R. Todorović, Nenad R. Filipović
Journal of Molecular Structure | DOI: 10.1016/j.molstruc.2023.135157
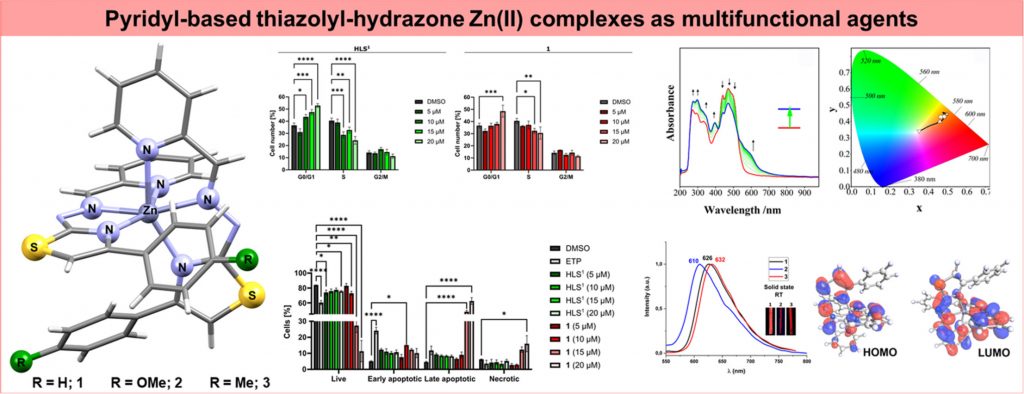
Thiazolyl-hydrazones (THs) exhibit a wide spectrum of biological activity that can be enhanced by complexation with various metal ions. Zn(II) complexes with α-pyridine-1,3-TH ligands may represent an alternative to the standard platinum-based chemotherapeutics. In addition, they show photoluminescence properties and thus can be regarded as multifunctional materials. In this study, we synthesized and characterized three neutral Zn(II) complexes (1–3) with pyridine-based TH ligands HLS1‒3 in order to investigate the influence of the ligands charge on the structure and intermolecular interactions in the solid state, and consequently photophysical properties. The deprotonation of the ligands mainly affects the relative energies of electronic levels in the complexes, compared to cationic counterparts, resulting in similar photoluminescence mechanisms and quantum yields with a small shift in emission energy. The influence of the substitution at the ligands’ periphery on the selected quantum molecular descriptors of the complexes is localized to the substitution site. Also, the substituents did not considerably influence the redox responses of the complexes. However, predominant spectral changes were observed in the course of the first reduction and oxidation processes which caused distinct spectral colour changes indicating their possible functionality for electrochromic applications. In addition, complex 1 showed antiproliferative activity with GI50 values below 2 µM on all tested cancer cell lines.
,
