Have a look at our new research article published in the CrystEngComm (IF: 3.756), an interdisciplinary collaboration with the research group of Nenad Filipovic and other research groups from University of Belgrad (Serbia) and with researchers from the Universidad de La Laguna (Tenerife, Spain), University of Novi Sad (Serbia), and Universidad de La Rioja (Spain).
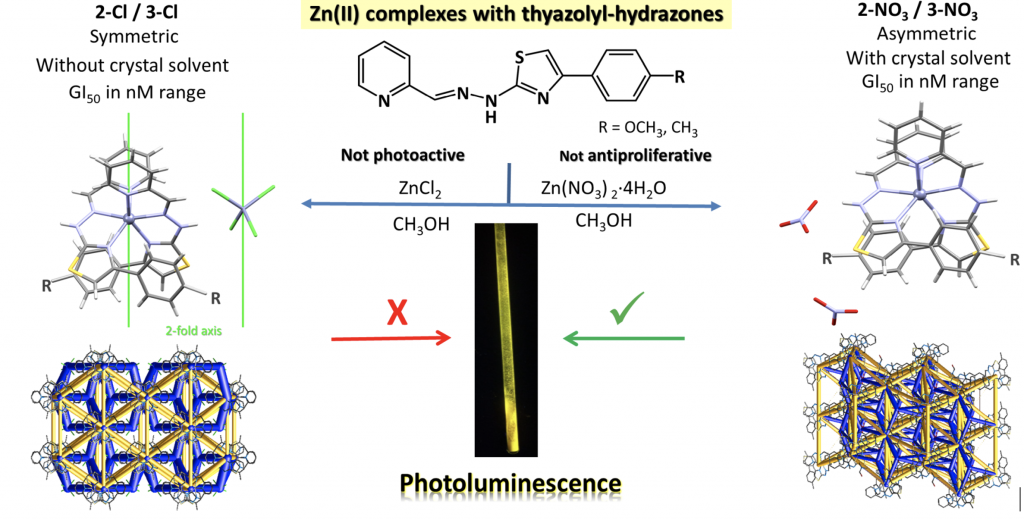
In this study, we describe the synthesis, characterization, and biological evaluation of a series of six zinc complexes (1–3-NO3 and 1–3-Cl) with pyridyl-based thiazolyl–hydrazone ligands with photoluminescent behavior. The in vitro antiproliferative activity of these compounds was evaluated against a panel of six human solid tumour cell lines. The study also included evaluation of their thermal and photophysical properties, cytotoxicity tests, and detailed crystal packaging analysis, corroborated by computational results, to establish structure–property relationships.
The relative ease of fine-tuning the thiazolyl–hydrazone moiety using a simple two-step synthetic procedure warrants further investigation of these complexes, especially their photo-physical and anticancer properties.
Zn(II) complexes with thiazolyl–hydrazones: structure, intermolecular interactions, photophysical properties, computational study and anticancer activity
Jovana B. Araškov, Aleksandar Višnjevac, Jasminka Popović, Vladimir Blagojević, Henrique S. Fernandes, Sérgio F. Sousa, Irena Novaković, José M. Padrón, Berta Barta Holló, Miguel Mongi, María Rodríguez-Castillo, José M. López-de-Luzuriaga, Nenad R. Filipović, Tamara R. Todorović
CrystEngComm | DOI: 10.1039/D2CE00443G
Abstract:
Earth-abundant, cheap and non-toxic zinc-based coordination compounds are drawing research attention as promising candidates for various applications, such as photoluminescent materials and anticancer agents. In this paper we report six zinc complexes (1–3-NO3 and 1–3-Cl) with pyridyl-based thiazolyl–hydrazone ligands, which differ in the nature of substituents at the ligands’ periphery, anion type, and geometry around the metal ion. The complexes were characterized by single-crystal and powder X-ray diffraction analysis, as well as IR and NMR spectroscopy. The symmetrical complexes 2-Cl and 3-Cl, where zinc atoms are located at a two-fold axis, do not exhibit photophysical properties, unlike their asymmetrical analogs 2-NO3 and 3-NO3 with the same complex cation. Asymmetrical pentacoordinated 1-Cl and hexacoordinated 1-NO3complexes exhibit photophysical properties. An admixture of allowed intra-ligand (1IL) and chloro (X)-to-ligand charge-transfer (1XLCT) electronic transitions is responsible for the fluorescence of the 1-Cl complex. The origin of the emission of the 1-NO3 complex is ascribed to an admixture of 3IL and ligand-to-ligand charge-transfer (3LLCT) forbidden electronic transitions, while for 3-NO3 most electronic excitations are of LLCT character. The thermal stability of the complexes is in accord with the strength of respective intermolecular interactions. The antiproliferative activity of the complexes was in the nanomolar range on some of the investigated cancer cell lines. Contrary to the increase of antiproliferative activity of the complexes in comparison to the free ligands in cancer cell lines, an acute toxicity determined in the brine shrimp assay follows the opposite trend. The overall results suggest that Zn(II) thiazoyl–hydrazone complexes have considerable potential as multifunctional materials.

