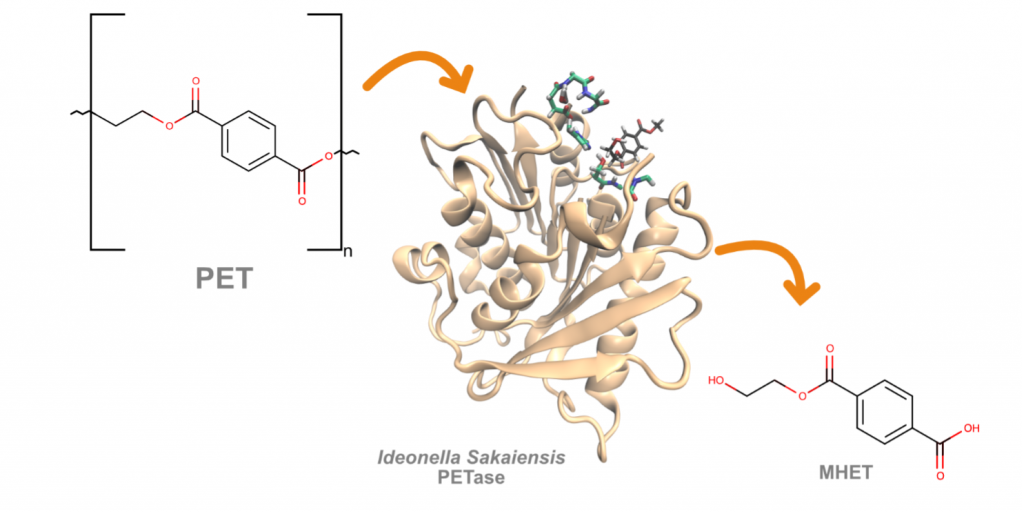
Please check our new research article recently published in Catalysis Science & Technology (IF: 6.119). In this study, we have solved with atomic level detail the catalytic mechanism of IsPETase, a plastic degrading enzyme secreted by Ideonella sakaiensis, a bacteria identified in a dumpster in Japan in 2016 that is capable of breaking down and consuming the plastic polyethylene terephthalate (PET) and to use it as both a carbon and energy source.
Our studies employing Quantum Mechanical/Molecular Mechanics simulations have revealed important structural and mechanist features for the activity of this enzyme. In particular, we have identified the critical role of a triad of residues composed by Ser207, Ile208, and Ala209 in stabilizing the catalytic essential aspartate residue. This suggests new strategies to further improve the activity of this enzyme in plastic PET degradation.
As this enzyme is a promising biocatalyst for the degradation of the PET polymers, the type of plastic most widely used for the fabrication of water bottles, these finding provide useful clues for the development of new and more environmentally friendly alternatives to the chemical methods of plastic recycling currently in use.
The critical role of Asp206 stabilizing residues on the catalytic mechanism of the Ideonella sakaiensis PETase
Rita P. Magalhães, Henrique S. Fernandes and Sérgio F. Sousa
Catalysis Science & Technology (2022) | DOI: 10.1039/D1CY02271G
Abstract:
Plastic accumulation is one of the main environmental issues of our time. In 2016, two enzymes capable of degrading Polyethylene terepthtalate (PET) , one of the most common plastic polymers, were discovered. IsPETase and IsMHETase, from Ideonella sakaiensis, work sequentially to degrade PET to its constituent monomers. In this work, the catalytic mechanism of IsPETase was studied by QM/MM. The reaction was found to progress in four distinct steps, divided in two major events: formation of the first transition intermediate and hydrolysis of the adduct. The transition state and respective reactant and product of each step were fully characterized and described. The rate limiting step was found to be step 2, with a cumulative energy barrier of 15.4 kcal/mol, which is in agreement with the experimentally determined kcat. Furthermore, in this study we have shown the critical role of a triad of residues composed by Ser207, Ile208, and Ala209 in stabilizing the catalytic aspartate residue. This finding confirms the importance of using a larger QM region, since our results disclose some important differences when compared with previous computational studies of the same mechanism. These results provide valuable insights on the catalytic mechanism of IsPETase that can contribute to the rational development of more efficient engineered enzymes.
