Please check our new research article recently published in ACS Catalysis (IF: 13.08). In this paper, we used QM/MM methods to unravel the catalytic mechanism of threonine aldolase with different substrates and through different catalytic paths.
Computational Studies Devoted to the Catalytic Mechanism of Threonine Aldolase, a Critical Enzyme in the Pharmaceutical Industry to Synthesize β-Hydroxy-α-amino Acids
Juliana F. Rocha, Sérgio F. Sousa, and Nuno M. F. S. A. Cerqueira
ACS Catalysis 2022 | DOI: 10.1021/acscatal.1c05567
Abstract:
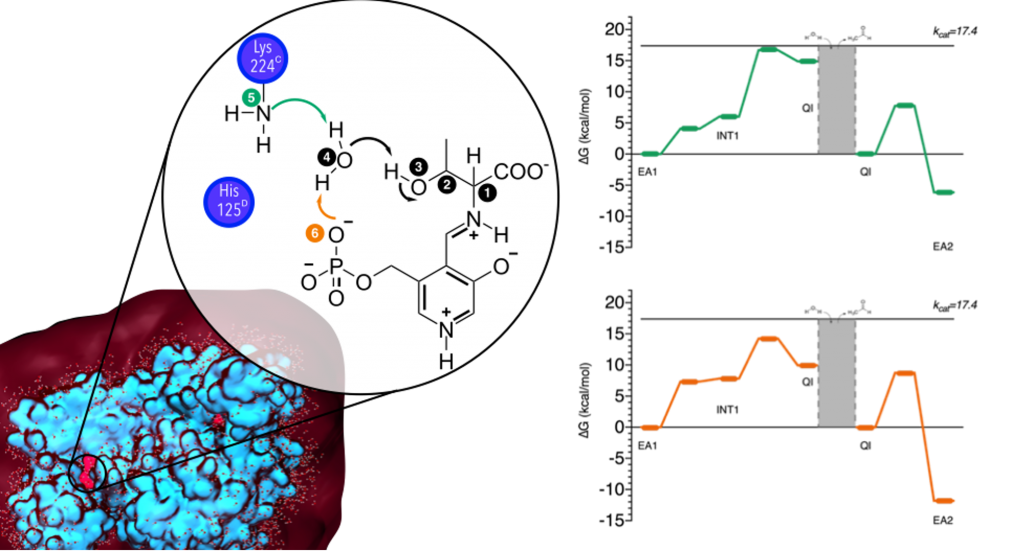
The catalytic mechanism of threonine aldolase (TA) was herein studied in atomic detail employing the computational ONIOM hybrid QM/MM methodology. TA is a PLP-dependent enzyme that catalyzes the retro-aldol cleavage of threonine into glycine and acetaldehyde, as well as the reverse reaction. This enzyme is currently seen as the optimal approach for the regioselective synthesis of β-hydroxy-α-amino acids (HAAs), which are very difficult to obtain by standard methods. The results obtained herein show that the catalytic mechanism of TA occurs in three steps: (i) deprotonation of the hydroxyl group of EA1, (ii) covalent bond cleavage, and (iii) hydrolysis. According to the Gibbs free energy profile, the rate-limiting step of the catalytic process is the covalent bond cleavage, which results in the formation of acetaldehyde. The calculated energy barrier for this step is 16.7 kcal mol–1, which agrees very well with the kinetic data available in the literature (17.4 kcal mol–1). All these results can now be used for the optimization of the synthesis of HAAs that serve as building blocks of several commercial drugs, such as antibiotics, immunosuppressants, and the anti-Parkinson’s disease drug L-threo-3,4-dihydroxyphenylserine.
