Please, check our recent publication in ChemPhysChem (IF 3.144):
An Unsual Cys‐Glu‐Lys Catalytic Triad is Responsible for the Catalytic Mechanism of the Nitrilase Superfamily: a QM/MM study on Nit2
Carla S. S. Teixeira, Sérgio F. Sousa, and Nuno M.F.S.A. Cerqueira
DOI: 10.1002/cphc.202000751 | ChemPhysChem
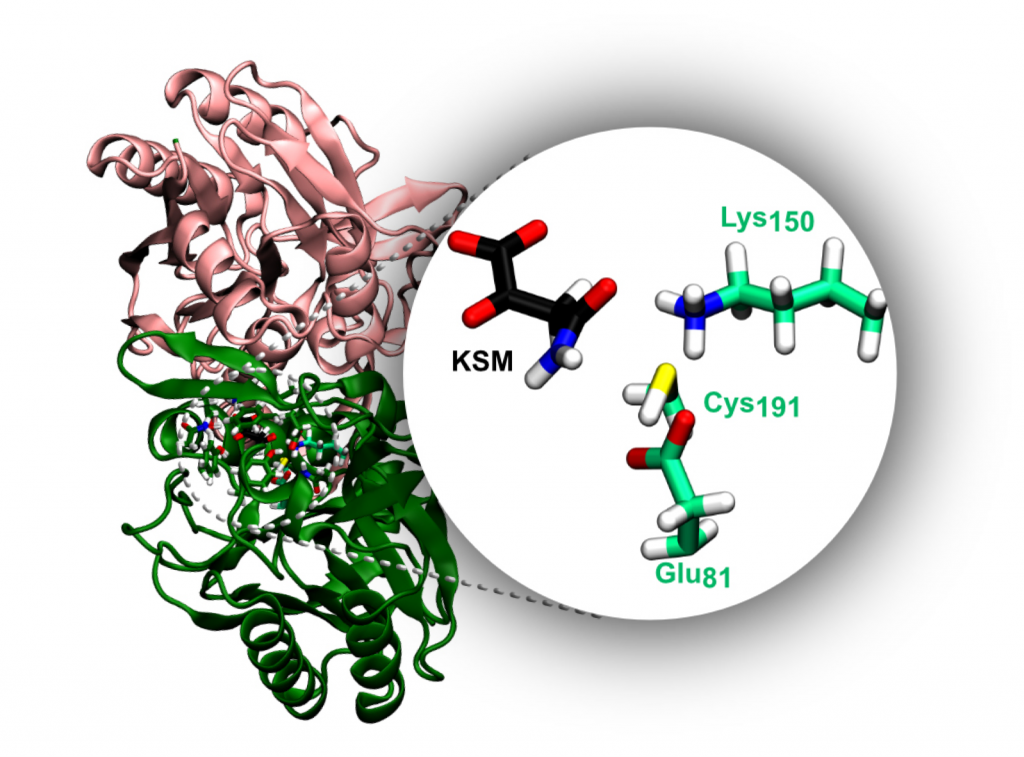
Nitrilase 2 (Nit2) is a representative member of the nitrilase superfamily that catalyzes the hydrolysis of α‐ketosuccinamate into oxaloacetate. It has been associated with the metabolism of rapidly dividing cells like cancer cells. This work reports the study of the catalytic mechanism of this enzymes through QM/MM. The results show that the catalytic mechanism of Nit2 employs a catalytic triad formed by Cys191, Glu81 and Lys150. Cys191 and Glu81 play an active role during the catalytic process while the Lys150 is shown to play only a secondary role. The results demonstrate that the catalytic mechanism of Nit2 involves four steps. The nucleophilic attack of Cys191 to the α‐ketosuccinamate, the formation of two tetrahedral enzyme adducts and the hydrolysis of a thioacyl‐enzyme intermediate, from which results the formation of oxaloacetate and enzymatic turnover. The rate limiting step of the catalytic process is the formation of the first tetrahedral intermediate with a calculated activation free energy of 18.4 kcal/mol, which agrees very well with the experimental k cat (17.67 kcal/mol).
