Please, check our recent publication in Inorganic Chemistry Frontiers, disclosing the complete catalytic mechanism of Xanthine Oxidase.
The complete catalytic mechanism of Xanthine Oxidase: a computational study
Pedro MG Ribeiro, Henrique S Fernandes, Luisa B. Maia, Sergio Sousa, José JG Moura and Nuno M. F. S. A. Cerqueira
DOI: 10.1039/D0QI01029D | Inorganic Chemistry Frontiers
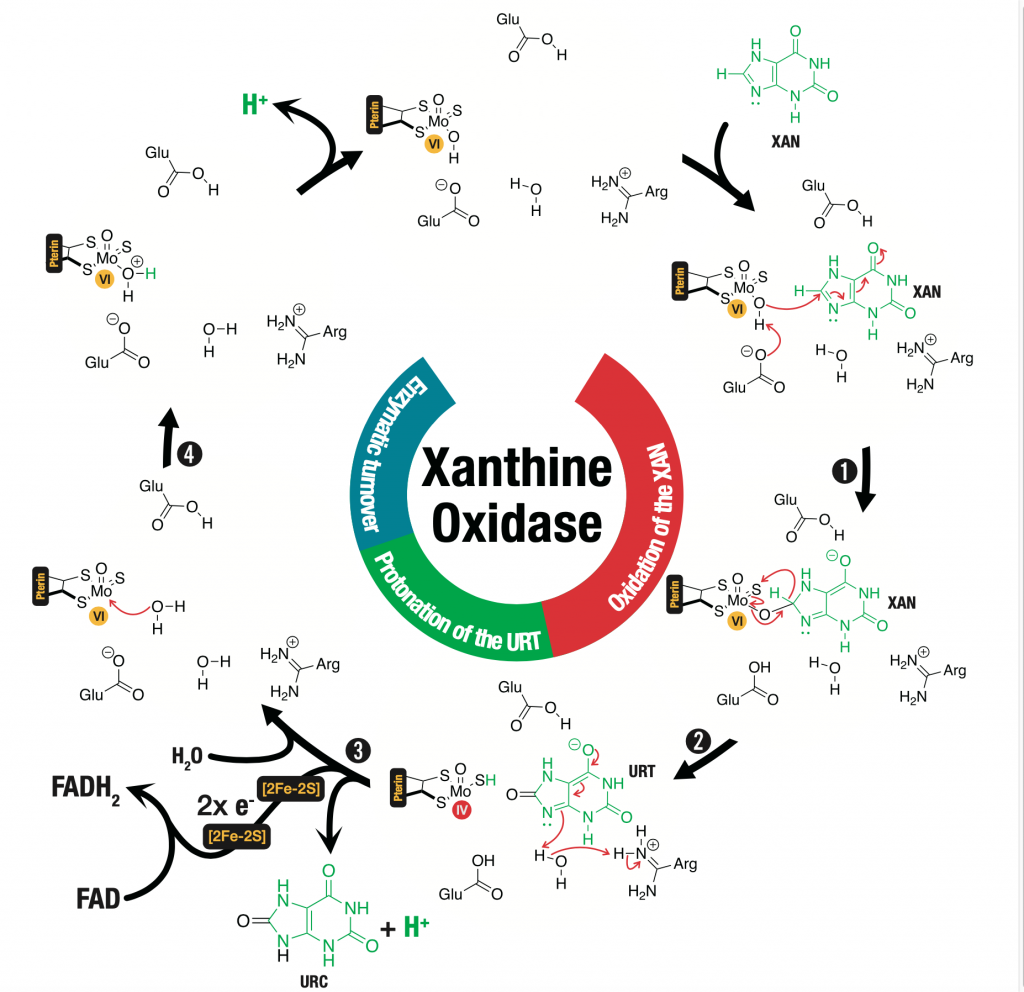
In this article, Quantum mechanical/molecular mechanical (QM/MM) methods were used to study the full catalytic mechanism of xanthine oxidase (XO). The XO catalyzes the conversion of xanthine (XAN) to uric acid (URC), requiring the presence of a molybdenum cofactor (Moco). The mechanism occurs through four reactional steps. Initially, the proton from the hydroxyl group of Moco passes to Glu1261 and the activated hydroxyl group makes a nucleophilic attack to XAN. Then, an hydride is transferred from the tetrahedral intermediate to the sulfur atom of the Moco, reducing Mo(VI) to Mo(IV). In third step, one molecule of URC is formed through its protonation by Arg880. Once this reaction is complete, FAD is reduced to FADH2, oxidizing the Mo(IV) to its initial oxidation state of Mo(VI). The enzymatic turnover is achieved with the reaction of one water molecule with the Moco. The rate-limiting step of the full catalytic mechanism is the hydride transfer that requires a free activation barrier of 16.9 kcal/mol, which closely agrees with the experimental kcat value (18.3 s-1), which corresponds to approximately 15.7 kcal/mol. This work also elucidates the key role played by Arg880 in the catalytic mechanism and the importance of Glu802 in the binding of the substrate. Both residues were previously shown to be important by mutagenesis studies, but their role was still not clearly understood. Additionally, it was observed the presence of a tunnel of water molecules located close to Moco and Glu1261 that is important for the enzymatic turnover. The determined transition state structures can now be used to help the development of transition-state analogs inhibitors targeting XO.
