Please check our latest collaborative paper published in ACS Catalysis on the determinants for the catalytic power of enzymes. This study, resulting from a collaboration with the Faculty of Sciences of the University of Porto and with the Institute of Biomedical Sciences, Academia Sinica (Taipei), involved the creation and analysis of a curated dataset of activation free energies, binding free energies and enzyme efficiency values for 934 enzymes. Despite the diversity of enzyme structures, substrates, chemical reactions catalyzed and environmental conditions, our study shows that enzymes have substrate binding free energies, activation free energies and catalytic efficiencies that fall in a narrow range of values. Different strategies that enzymes employ to increase their efficiency are identified and discussed, yielding important clues regarding the reasons for the catalytic power of enzymes.
Activation Free Energy, Substrate Binding Free Energy, and Enzyme Efficiency Fall in a Very Narrow Range of Values for Most Enzymes
Sérgio F. Sousa, Ana R. Calixto, Pedro Ferreira, Maria J. Ramos, Carmay Lim, and Pedro A. Fernandes
DOI: 10.1021/acscatal.0c01947 | ACS Catalysis
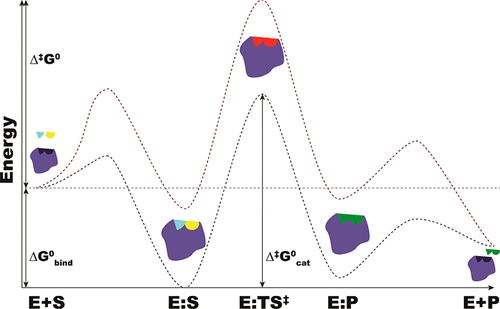
Enzymes are responsible for controlling many biological processes, catalyzing different reactions using substrates with diverse complexity, size, and chemistry. How enzymes work and achieve their catalytic efficiency are some of the fundamental questions of life. Most studies have focused on specific enzymes to elucidate their reaction mechanisms and rate acceleration. No study, to our knowledge, has performed systematic analyses of the available properties of all six classes of enzymes. Here, we have systematically analyzed three parameters pertinent to enzyme catalysis, viz. activation free energy, substrate-binding free energy, and enzyme efficiency, which were estimated using the turnover number and the KM value at a given temperature for each enzyme. By correlating these parameters with structural/biological information, including the presence/absence of inorganic and/or organic cofactors, the enzyme’s oligomerization state, the monomer size, the cellular location, and the substrate specificity/promiscuity, we show that, despite the chemical, structural, and reaction diversity of the enzymes analyzed, the activation free energies, substrate-binding free energies, and enzyme efficiencies of most enzymes fall in a very characteristic narrow range of values. Various strategies are used by enzymes to confine the values of the catalytic parameters to such a narrow range. These include their location, their monomeric size or oligomeric state, their specificity, the environmental conditions, and the use of cofactors.
