Please check our latest collaborative paper published in ACS Catalysis on the Catalytic Mechanism of Human Aldehyde Oxidase, solved by QM/MM methods, resulting from a collaboration with the Universidade Nova de Lisboa and the Faculdade de Ciências da Universidade do Porto.
Catalytic Mechanism of Human Aldehyde Oxidase
Pedro Ferreira, Nuno M. F. Sousa A. Cerqueira, Pedro Alexandrino Fernandes, Maria João Romão, and Maria João Ramos
DOI: 10.1021/acscatal.0c02627 | ACS Catalysis
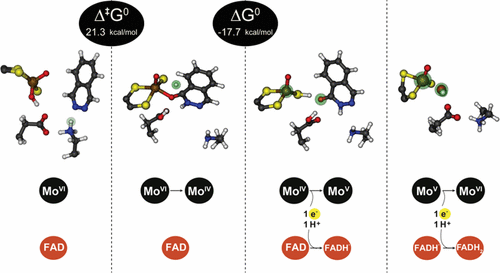
The mechanism of oxidation of N-heterocycle phthalazine to phthalazin-1(2H)-one and its associated free energy profile, catalyzed by human aldehyde oxidase (hAOX1), was studied in atomistic detail using QM/MM methodologies. The studied reaction was found to involve three sequential steps: (i) protonation of the substrate’s N2 atom by Lys893, (ii) nucleophilic attack of the hydroxyl group of the molybdenum cofactor (Moco) to the substrate, and (iii) hydride transfer from the substrate to the sulfur atom of the Moco. The free energy profile that was calculated revealed that the rate-limiting step corresponds to hydride transfer. It was also found that Lys893 plays a relevant role in the reaction, being important not only for the anchorage of the substrate close to the Moco, but also in the catalytic reaction. The variations of the oxidation state of the molybdenum ion throughout the catalytic cycle were examined too. We found out that during the displacement of the products away from the Moco, the transfer of electrons from the catalytic site to the FAD site was proton-coupled. As a consequence, the most favorable and fastest pathway for the enzyme to complete its catalytic cycle was that with MoV and a deprotonated SH ligand of the Moco with the FAD molecule converted to its semiquinone form, FADH•.
