Total stereo-selective Michael Addition of N- and S- nucleophiles to a D-erythrosyl 1,5-lactone derivative. Experimental and theoretical studies devoted to the synthesis of 2,6-dideoxy-4-functionalized-D-ribono-hexono-1,4-lactone
Juliana F. Rocha, David S. Freitas, Jennifer Noro, Carla Sílvia Silva Teixeira, Cristina E. A. Sousa, M. Jose Alves, and Nuno M. F. Sousa A Cerqueira
10.1021/acs.joc.8b00769 |The Journal of Organic Chemistry
Abstract
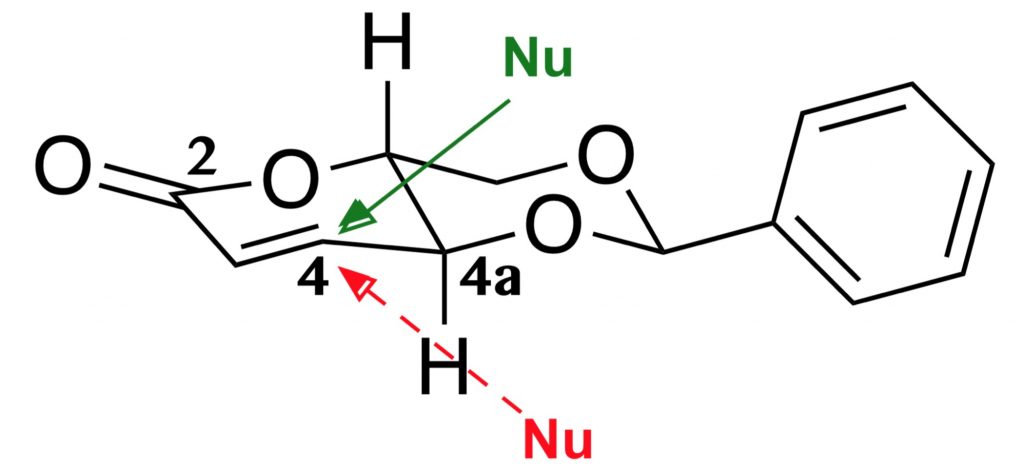
The synthesis of a 1,5-lactone 2,4-O-alkylidene-D-erythrose derivative was found to be a highly stereoselective template in Michael addition trough the reaction of a D- erythrosyl 1,5-lactone derivative with nitrogen and sulfur nucleophiles. The sulfur adducts formed are 1 (D-erythrose derivative):1 (nucleophile), and the nitrogen adducts are 1:2. Both were then treated under HCl to give 2,6-dideoxy-4-functionalized-D- ribono-hexono-1,4-lactone by a reaction cascade in high overall yield. Reaction’s scale up even improves the yield.
The theoretical and computational results clearly explain the origin of the stereoselectivity, and the energetic course of reactions starting with nitrogen and sulfide nucleophiles.
Considering that the 1,4-lactones obtained in this work offer a new molecular scaffold for organic synthesis, these new results provide a solid theoretical platform that can be used to speed up synthesis of other derivatives in a stereo- andregio- selective way.
